Hey Guys!
We hope you guys doing good today. We are going to discuss Photoelectric effect class 12. We shall discuss in detail below, before that short introduction to the Photoelectric effect. The electrons are emitted from the metal surface when the light is incident on surface this phenomenon is known as Photoelectric effect.
Everyone should know about Photoelectric effect. In the Topic Photoelectric effect class 12, we are going to discuss Define photoelectric effect? What are the laws of Photoelectric emission?
Photoelectric effect class 12 notes prepared from book Atomic and Nuclear physics. If anyone need a notes, you can find download button to download photoelectric effect class 12 notes.
Photoelectric effect class 12
Define Photoelectric Effect?
Heinrich Hertz in 1887 discovered that a metallic surface is capable of emitting electrons once light of very short wavelength falls on it. He noted that the air within the spark gap became a better conductor when it was illuminated by ultraviolet light from an arc lamp. Hallwachs in 1888 found that:
- when ultra-violet light was incident on a neutral zinc plate, the plate became positively charged.
- when ultra-violet light was incident on a negatively charged zinc plate, it lost its charge rapidly, and
- when ultra-violet light was incident on a positively charged zinc plate, it became more positively charge
He came to the conclusion that only negatively charged particles can be emitted by the surface under the action of ultra-violet light. Thomson in 1898 showed that the e/m value of emitted particles was the same as that for cathode rays. Einstein in 1916 studied the effect of visible light of a range of frequencies on sodium, potassium, cesium, rubidium and lithium and located that the electrons were ejected out of those metals.
The electrons ejected out of the metal under the action of light are known as photoelectrons and this phenomenon is known as ‘photoelectric effect’
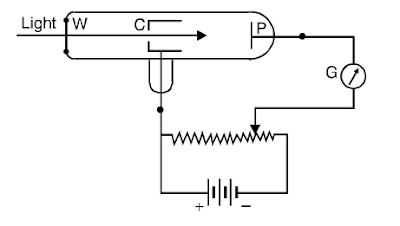
Experiment. Photo-electric effect is studied with the help of the following experiment. An evacuated glass tube has a photo-electrically sensitive plate P. It may be a highly polished zinc plate. A hollow cylinder C has a small hole that permits the incident light to fall on the plate P. A quartz window W allows the light to enter the tube. P is connected to the negative and C is connected to the positive terminal of the battery. A sensitive galvanometer is used in the circuit as shown in (Fig. 1).
When light from some source such as mercury arc lamp falls on the plate P, photoelectrons are ejected out of the plate. These photoelectrons are attracted by the positively charged cylinder C. The galvanometer shows deflection and thus photo-electric current is produced in the circuit. The effect of light of a range of frequencies was studied. Based on the results obtained, the following laws of photo-electric emission were formulated.
What are the Laws of Photoelectric Effect?
- For a given metallic surface, there is a minimum frequency for which the incident light can eject the photoelectrons out of the metal. Light of frequency smaller than this particular value cannot eject electrons, no matter how long it falls on the surface or how high is its intensity.
- The number of photoelectrons ejected depends upon the intensity of the incident light. Thus, the photo-electric current depends upon the intensity of the incident light.
- Light of frequency higher than the critical frequency or threshold frequency ejects electrons of different velocities. The maximum velocity with which an electron is ejected, depends upon the frequency of the incident light and not on the intensity of the incident light. The threshold frequency is the minimum frequency which can eject an electron out of the metal. Light of frequency less than the threshold frequency cannot eject an electron out of the metal, howsoever intense the light may be.
- The maximum kinetic energy of the ejected electrons increases linearly with the frequency of the incident light. It does not depend on the intensity of the incident light.
The above experimental facts could not be explained on the basis of the wave theory of light. Einstein, in 1905, explained the photo-electric effect based on Planck’s idea of quantum theory of light. According to quantum theory, light consists of quanta or corpuscles of energy hv which move through space with the velocity of light. These particles are known as photons and each photon has an energy = hv, where h is the Planck’s constant and v is the frequency of light. When a photon collides with an electron in the metal, it may transfer energy to the electron. This transfer is an “all or none” process, i.e., either the photon gives the whole of its energy hv to the electron or no energy to the electrons. We hope you find helpful from this post on Photoelectric effect class 12.
Reference:
Book referred for this post: Atomic and Nuclear Physics - N. SUBRAHMANYAM
https://books.google.com/books?id=yqFbehAUbhAC
*Disclaimer:
"Laws of the Photoelectric effect written same as in the book. We can't write the laws as per we want. If one word change, total meaning of passage leads to misunderstand. So, we handled with care."


0 Comments
Chat with InfoWite!