The uncertainty principle is valid for - In the experiments for the determination of specific charge of an electron, it was shown that electron gets deflected by the application of electron and magnetic fields. It means that the electron behaves as a particle, and it has definite mass, momentum and energy which can be measured with desired accuracy. But the experiment of Davisson and Germer, and Thomson showed clearly the wave nature of electron and the wavelength of the electron is given by
Here h is the Planck’s constant and mv is the momentum of the electron. A wave extends through space, and it is difficult to locate the exact position of an electron behaving as a wave, at any given instant of time.
This dual behaviour of electron as a particle as a wave presents difficulty in locating the exact position and momentum at the same time. This difficulty is overcome according to Heisenberg’s uncertainty principle.
This principle states that the exact position and momentum of a particle (say electron) cannot be determined simultaneously with a desired accuracy. Taking as the error in determining its position andthe error in determining its momentum at the same instant, these quantities are related as follows:
The product of the two errors is approximately of the order of Plank’s constant. Multiplying and dividing the left-hand side of equation by v, the velocity of the particle
Here E represents the uncertainty in the measurement of the energy of a particle and t the uncertainty in the measurement of time.
In equation, the product is of the order of h/ 2. If x is small, p will be large and vice versa. It means that if one quantity is measured accurately, the other quantity becomes less accurate. A similar argument holds good for E t also.
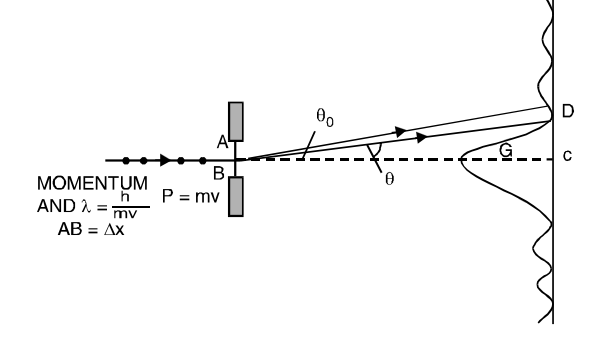
Consider a beam of electrons travelling in the direction shown in Fig.1 above the slit AB of width x is perpendicular to the path of the electrons. Before entering the slit, the electron has a definite momentum p = mv. After passing through the slit the electron gets diffracted and acquires a momentum along OG. The angular deflection θ depends upon the component of the momentum parallel to the slit, i.e., p = p sinθ = pθ for small angular deflection. The angle θo corresponding to the direction of first minimum of the diffraction pattern is
θo = λ x for small value of θo
(or)
λ θo
λ θθo
But
λ = h
= h θθo
Taking,
θ = θo Approximately,
Therefore,
The probable deflection θ of the electron is less than θo and according to Heisenberg, the uncertainty relationship is
In general, any experiment that measures position or momentum accurately, introduces an uncertainty in the other. The uncertainty is never less than the predicted value according to Heisenberg’s relation. Thus, any instrument cannot measure the quantities more accurately than predicted by Heisenberg’s principle of uncertainty or


0 Comments
Chat with InfoWite!